
About
Dr. Manfred Keller, founder of Keller Pharmaconsult, is specialized in inhalation devices and drug formulations for pulmonary and nasal drug delivery and targeting.
There is substantial expertise in all development steps until approval comprising of chemistry (formulation development), control, manufacturing (CMC-aspects), pharmacokinetics and clinical testing meeting with current regulatory requirements for inhaled drug device combinations.
Support will be given selecting the best possible device (MDI, DPI, nebulizer) including formulation for an optimal positioning of a new inhalation product in a competitive market.
The objective is: Adding value to Your business
Service Offering
Service and experties aiming for a win-win for pharmaceutical development companies, investors, patients, health care providers and payers
Early stage advice and feasibility
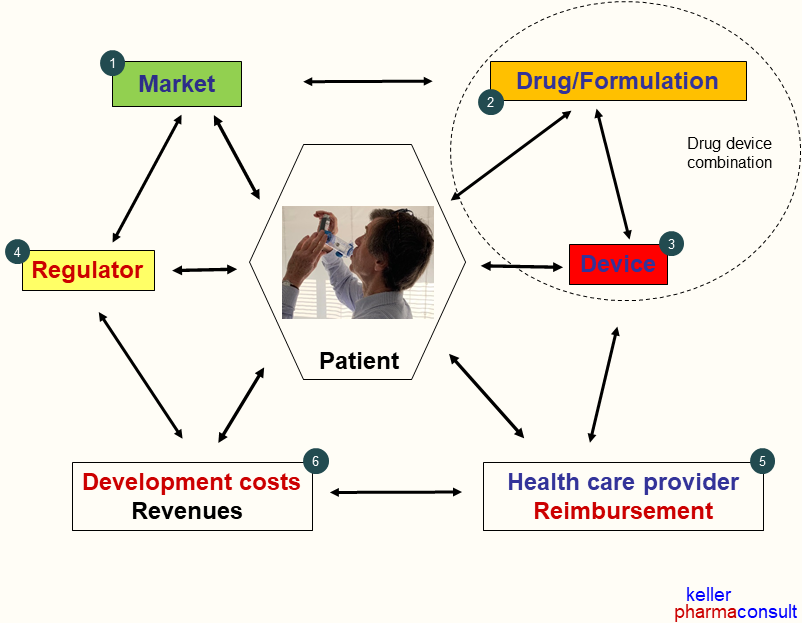
- Which drugs and features will qualify for inhaled drug delivery? (1) (2)(3)
- Evaluating the patent landscape and assessing the potential for freedom to operate. (1) (2)(3)
- What must be considered for inhaled drug delivery concepts and systems?
(1) – (6) - Which delivery system (MDI, DPI, nebulizers) will be suitable for a distinct project? (2)(3)
- How to conduct feasibility tests to develop and/or select the most feasible drug device combination? (2)(3)(5)
- Suitable in-vitro and in-vivo tests for drug device differentiation and selection. (2)(3)(5)(6)
- Planning of tailored product development steps considering realistic timelines and budgets (2)-(6)
- What can be achieved in a realistic timely and cost-effective manner? (1)-(6)
- What is important to approve a drug-device combination for inhalation? (1) – (6)
- What must be considered for a new product in a competitive therapeutic field and market? (1) – (6)
Regulatory aspects and guidelines for orally inhaled products (OIPs)
- Regulatory pathways, (NCEs, hybrids, generics, orphan drug status). (4)
- Demands differing between Europe and the USA for NCEs, generics and orphan drugs (1)(4)
- Medical device characterization and registration in the EU and USA, (1) (3) (4)
- Advice on chemistry, manufacturing, and controls (CMC), (2)(3)(4)
- In-vitro and in-vivo tests needed to complete the development pharmaceutics section, (2)(3)(4)(5)
- Regulatory expectations on safety- and efficacy aspects (phases I-IV), (4)
- Demands on clinical and pharmacokinetic studies for demonstration of bioequivalence and/or therapeutic efficacy compared to standard therapy. (4)
- Support on medical advice with regulatory bodies.(4)(6)
Input on device and formulation for optimized drug delivery and targeting
- Disease and patient specific requirements and hurdles, (1) – (6)
- Identification and selection of devices including risk assessment, (2)(3)
- Definition of development objectives and preliminary product specifications, (2)-(5)
- Manufacturing and scale-up of formulation and device, (2)-(5)
- Cost of goods (CoGs) analysis (1)(2)(3)(5)(6)
- Patient needs, skills, preferences on drug device configurations, (2)(3)(6)
- Handling and simulated use tests (SUT), (2)(3)(5)
- Hygiene aspects and cleaning, (1)-(4)
- Pricing and reimbursement. (1)(2)(3)(5)(6)
Preparation of expert opinion reports on:
- Device and drug formulations for nasal and pulmonary inhalation (1) (2)(3)(4)
- Regulatory expectations, potential hurdles and pitfalls (2)(3)(4)(5)
- Strategic consideration and concepts to become successful in a competitive market (1)(3)(4)(5)(6)
- Risk assessment and gap analyses (1)-(6)
Who is Dr. Manfred Keller
Click below to see an extract of patents Dr. Manfred Keller has worked on.

See a detailed curriculum vitae from the founder of pharmaconsult.

Click below to see a list of publications Dr. Manfred Keller has published.
